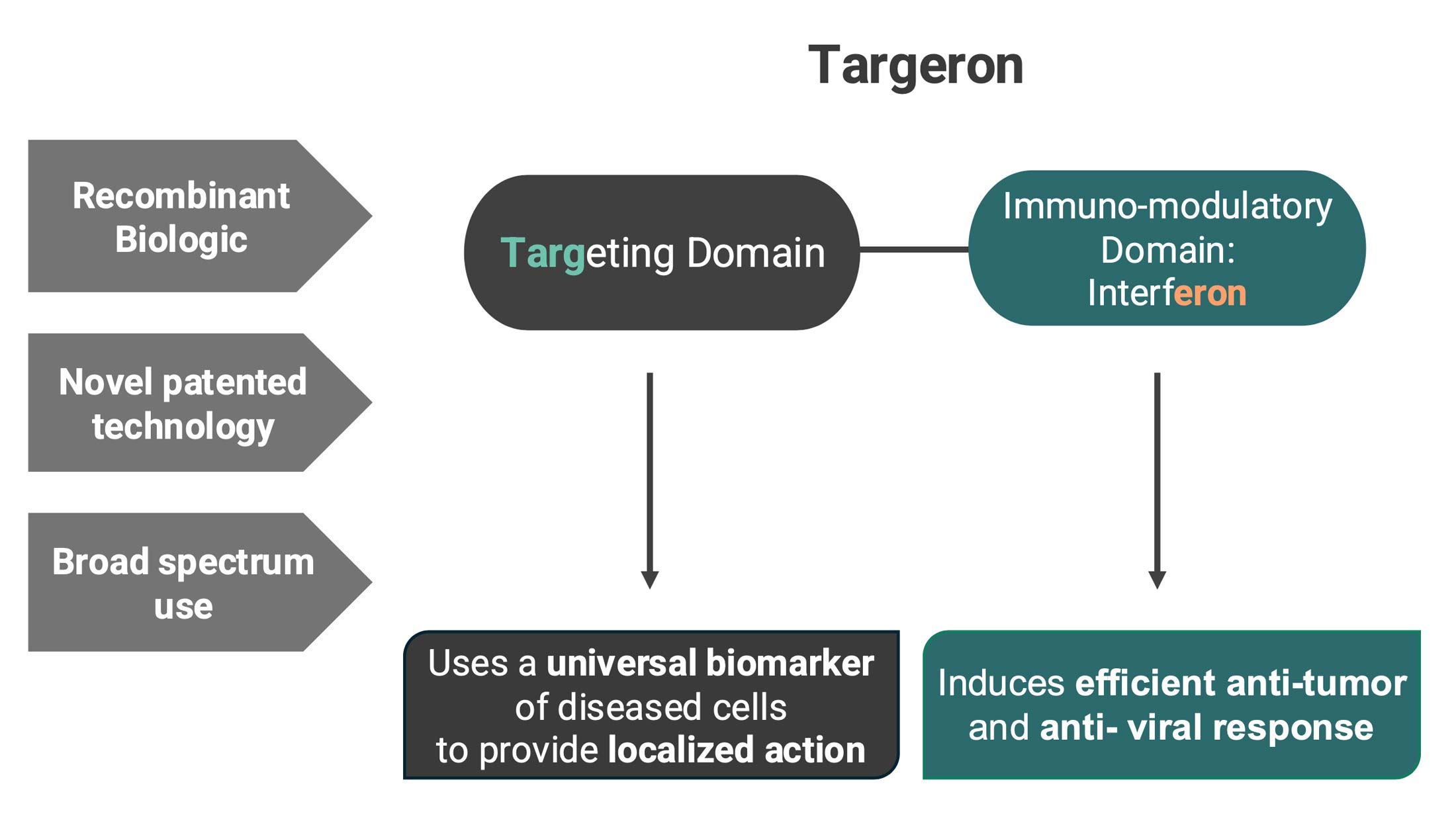
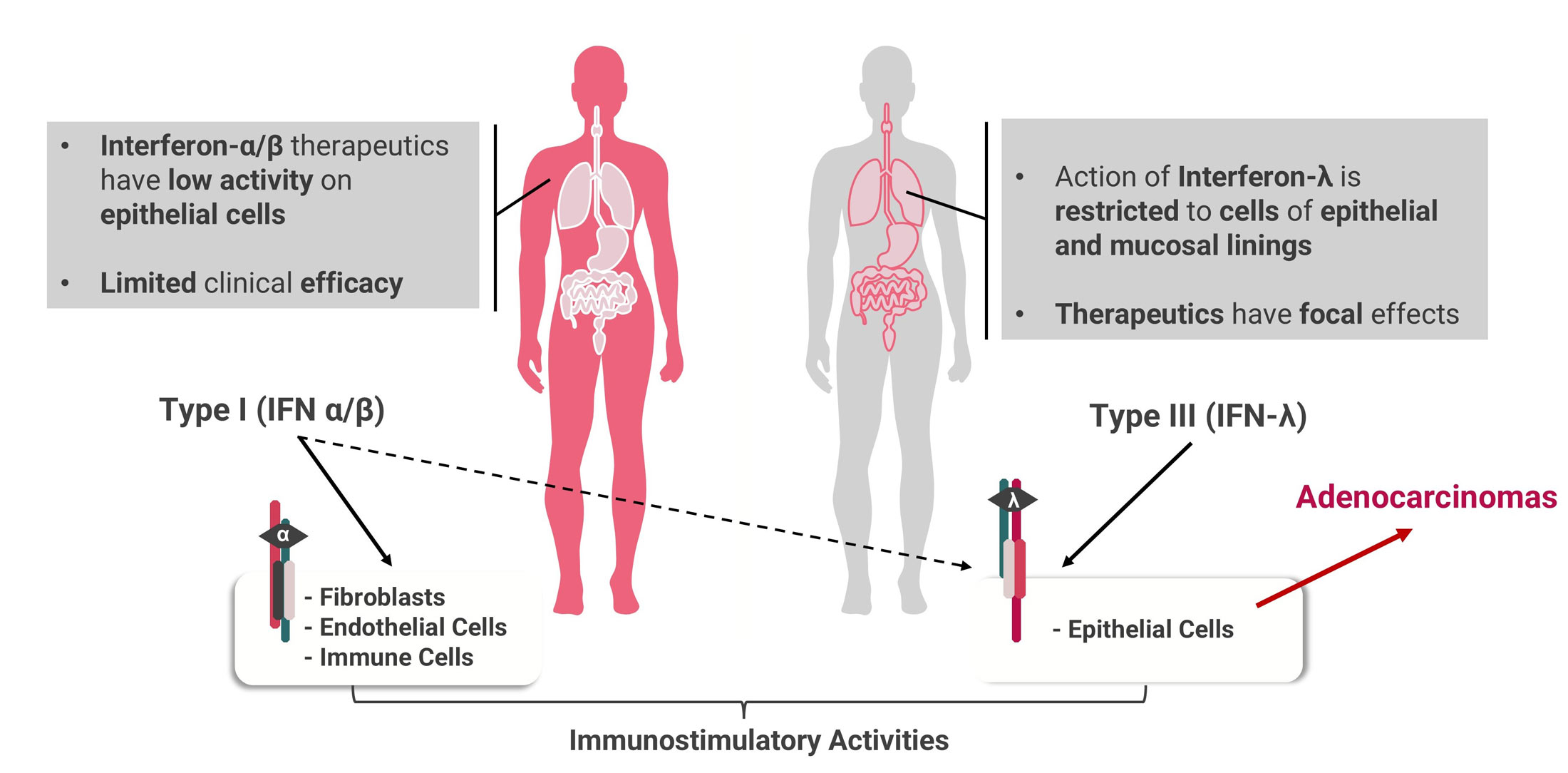
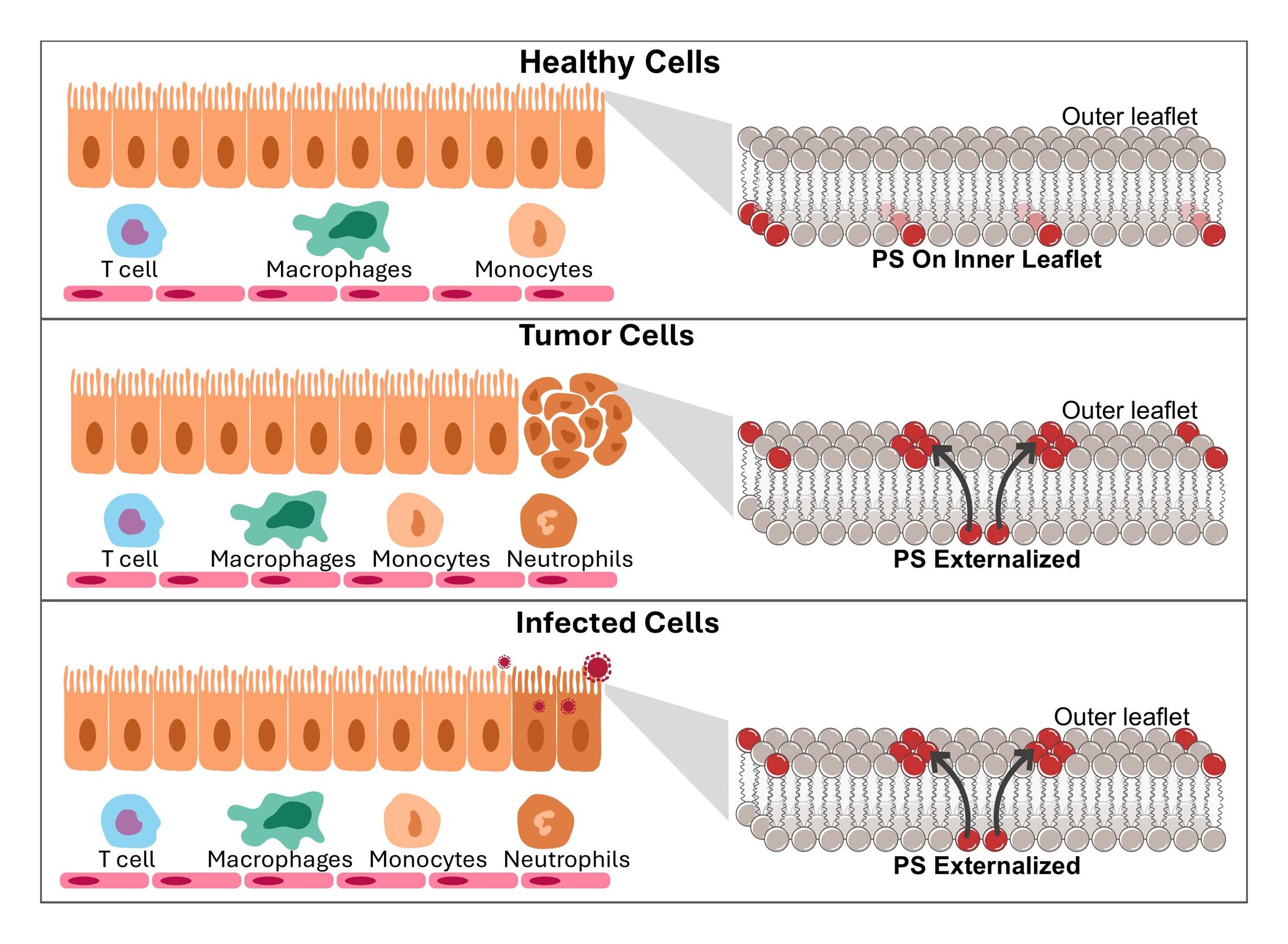
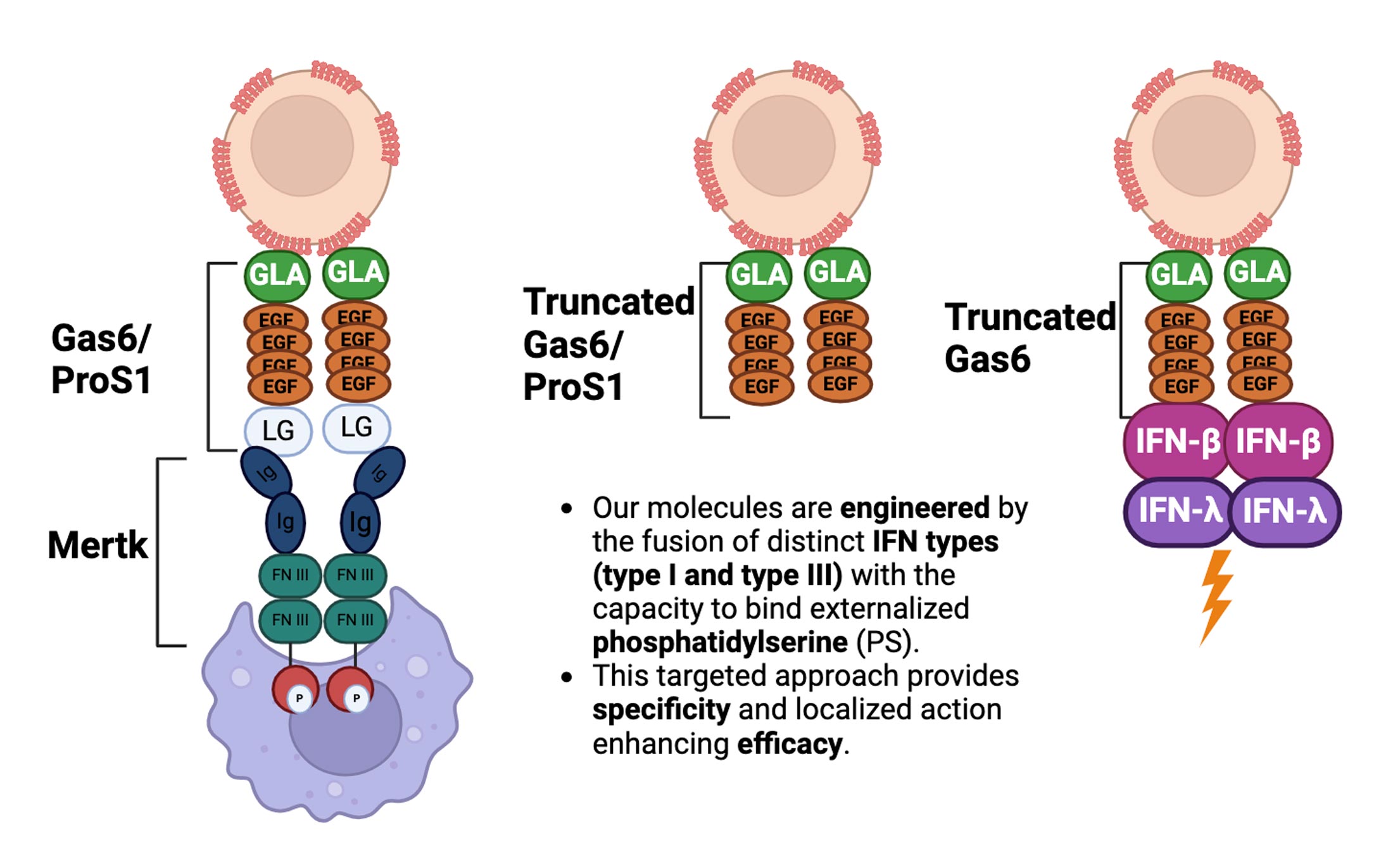
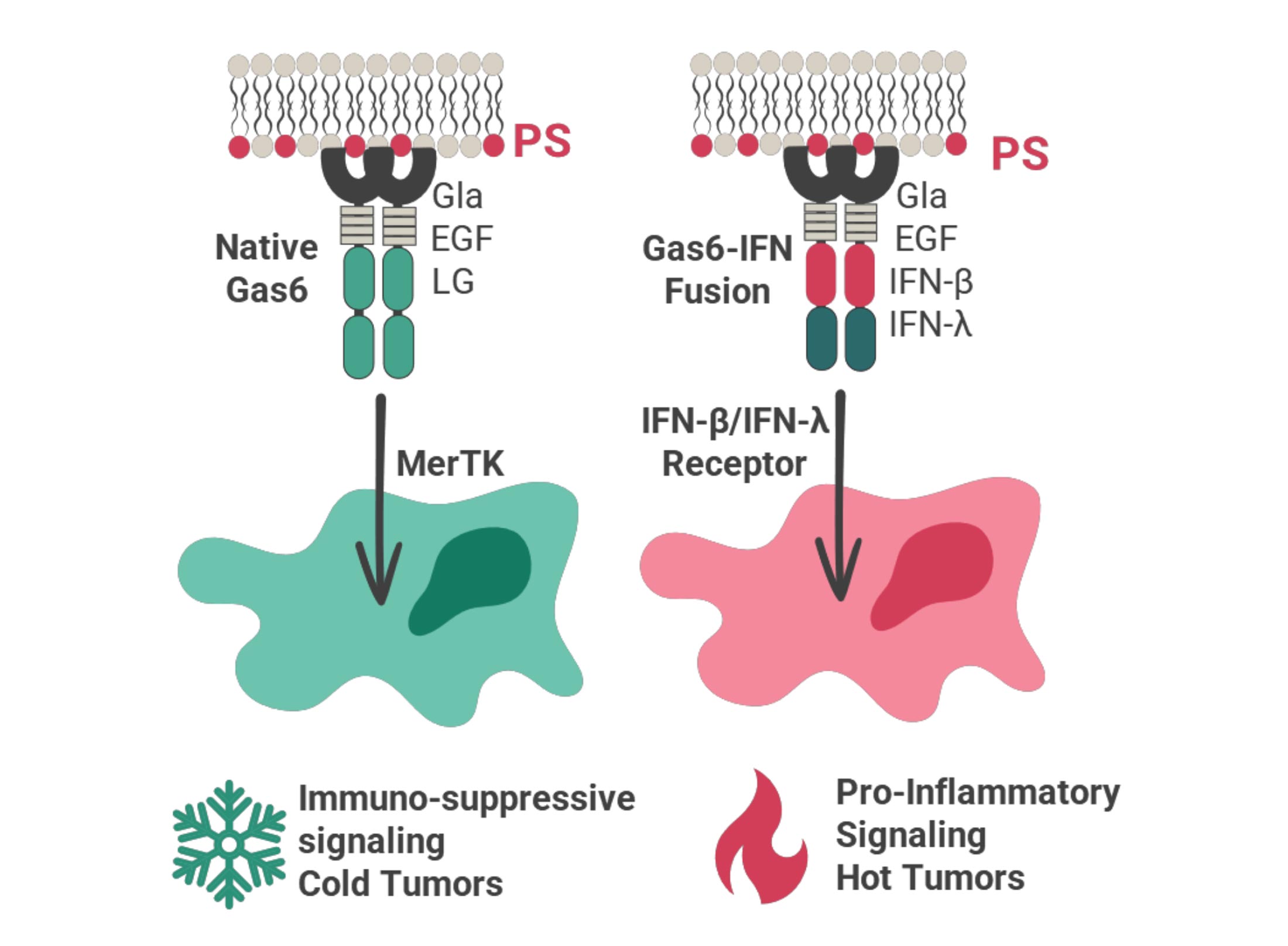
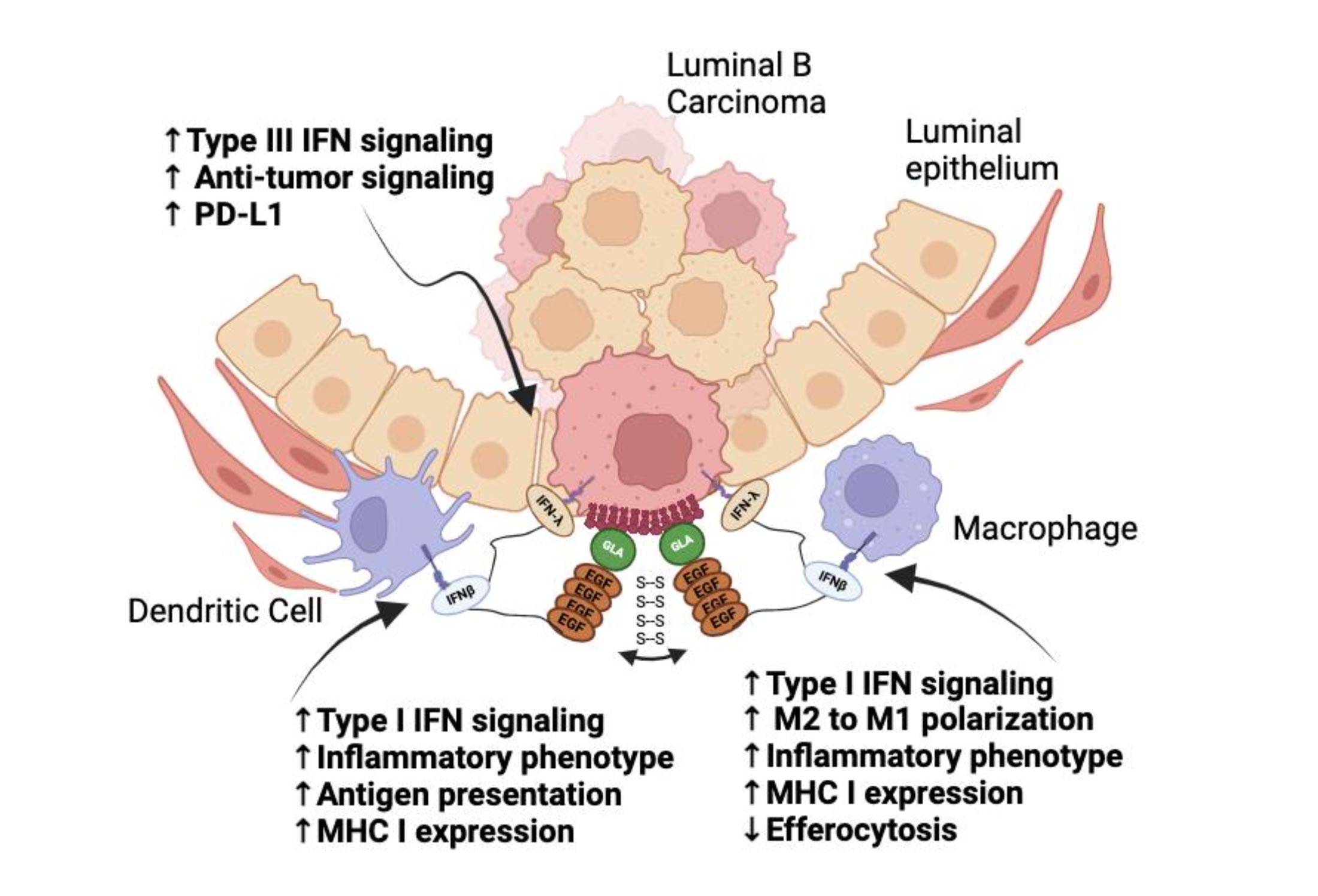
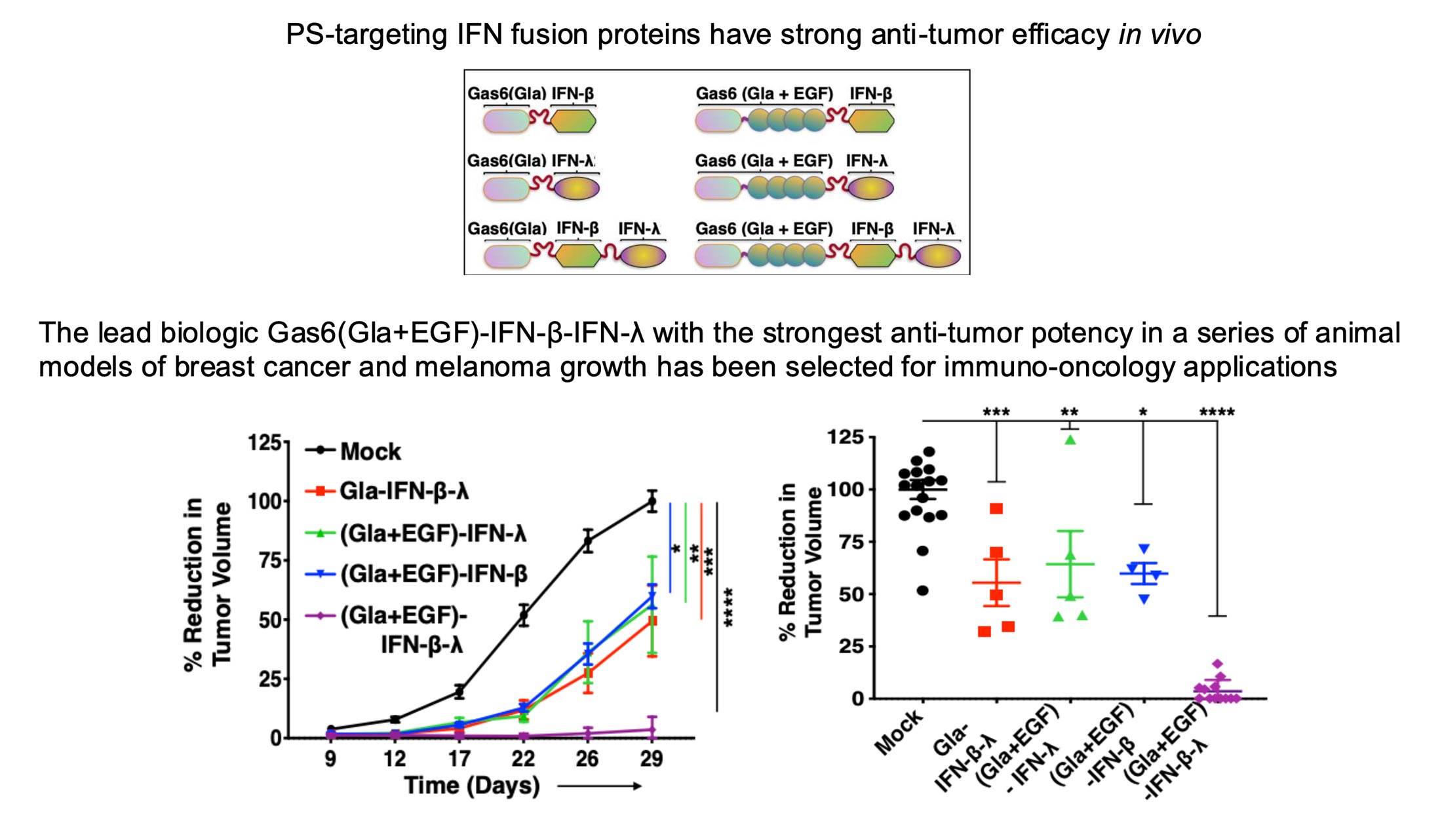
Targeron Technology
Targeting Interferons to Externalized Phosphatidylserine on Diseased Cells
Animation: PS-Targeting Interferon by Targeron Therapeutics
Explainer video on how Targeron’s innovative technology combines PS-targeting molecules with Type I and Type III interferons
Discover how Targeron Therapeutics is advancing cancer treatment through a novel immunotherapy that targets phosphatidylserine (PS), a universal immune evasion signal exposed in the tumor microenvironment. Animation by www.pix-videos.com